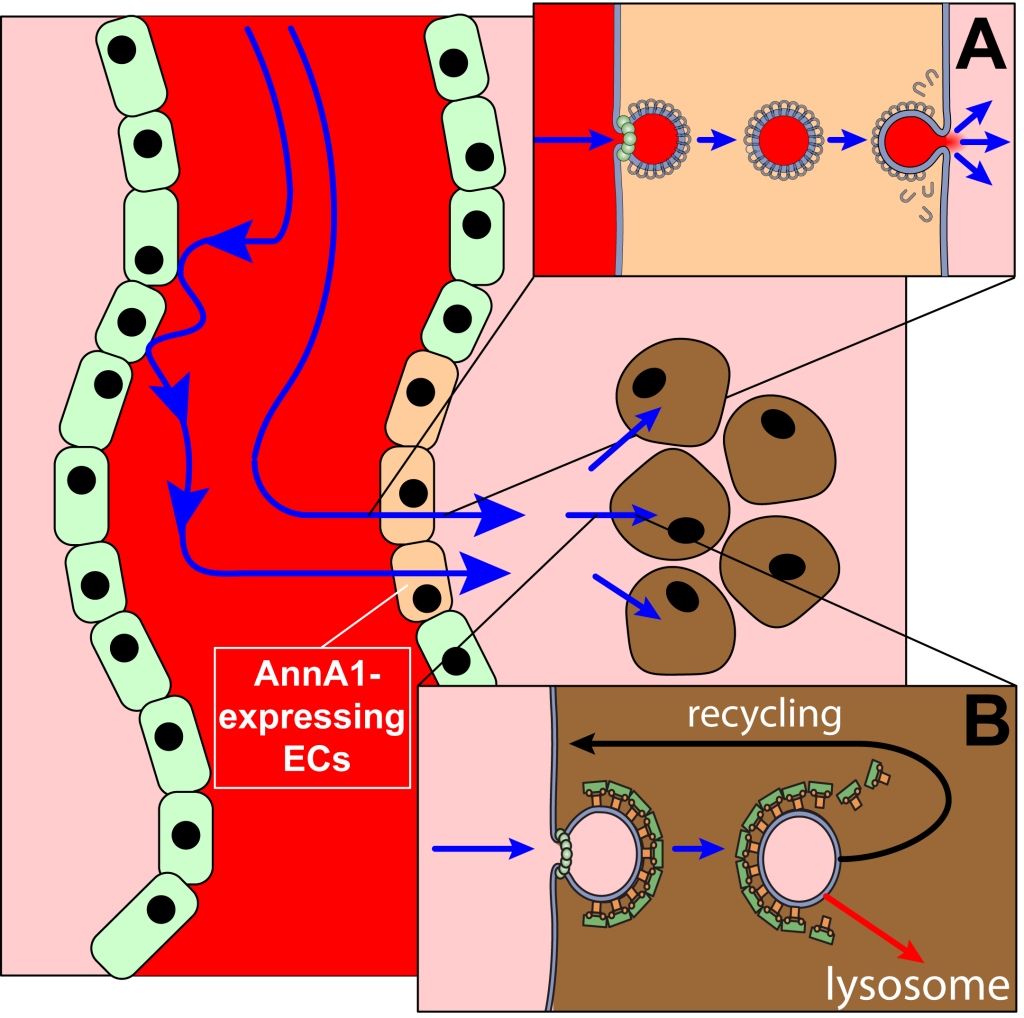
Vesicular transport networks have long been viewed as a promising route for drug delivery to a variety of high-value therapeutic targets, although so far very few such strategies have resulted in acceptable outcomes in the clinical setting. One of the reasons for this mixed record is the frequently ignored need to actively manipulate the interactions of transport proteins with their cognate receptors in order to enable their precise routing within the cell. Also frequently overlooked is the inability of a large number of vehicles to traverse multiple physiological barriers en route to the therapeutic target, a challenge that can be addressed by developing multi-target delivery systems. Endothelial cells in blood capillaries form a barrier which frequently poses a formidable challenge vis-a-vis effective targeting of anti-cancer medicines to the tumor site. Fortunately, dramatic progress was made in the past decade in deciphering the mechanisms of intra- and trans-cellular vesicular transport, which provides new opportunities for designing efficient and safe targeted drug delivery schemes. We are working in close collaboration with a group of medicinal chemists on designing a multi-component delivery system that can effectively localize to the solid tumor site even in the absence of leaky vasculature, and be internalized by the cancer cell via transferrin receptor-mediated endocytosis. This feature enables robust internalization of the vehicle by cancer cells, followed by its efficient routing to lysosomes. Thus, the new multi-target delivery system will be capable of not only crossing the endothelial barriers at the tumor site, but also selectively entering the cancer cells and delivering the payload to a specific cellular compartment (lysosomes) in order to disrupt microtubules with mertansine-based cytotoxins or inhibit enzymes involved in the remodeling of extracellular matrix, a critical component of cancer progression. To achieve the ambitious goals of this project, we are developing a new mass spectrometry-based strategy to study kinetics of protein/receptor interactions which control the routing of the carrier proteins through the vesicular networks. We are also developing a novel highly sensitive and selective technique for tracing carrier proteins and payloads in complex biological matrices using multi-metal coding and inductively coupled plasma (ICP) MS detection. The results of this work will be invaluable for guiding the design of effective targeted drug delivery strategies not only in oncology, but also in fields ranging from regenerative medicine to neurodegenerative disorders.
Related publications
1. C. Ren, C.E. Bobst, and I.A. Kaltashov. Exploiting His-tags for absolute quantitation of exogenous recombinant proteins in biological matrices: ruthenium as a protein tracer. Anal. Chem. 2019, 91, 7189-7198
2. S. Xu and I.A. Kaltashov. Evaluation of gallium as a tracer of exogenous hemoglobin-haptoglobin complexes for targeted drug delivery applications. J. Am. Soc. Mass Spectrom., 2016, 27, 2025-2032
2. H. Zhao, S. Wang, S.N. Nguyen, S.G. Elci and I.A. Kaltashov. Evaluation of non-ferrous metals as potential in vivo tracers of transferrin-based therapeutics. J. Am. Soc. Mass Spectrom. 2016, 27, 211-219
3. S. Wang, C.E. Bobst and I.A. Kaltashov. A new LC-MS based method to quantitate exogenous recombinant transferrin in cerebrospinal fluid: a potential approach for pharmacokinetic studies of transferrin-based therapeutics in the central nervous system. Eur. J. Mass Spectrom. 2015, 21, 369-376
4. S.N. Nguyen, C.E. Bobst, I.A. Kaltashov. Mass spectrometry-guided optimization and characterization of a biologically active transferrin-lysozyme model drug conjugate. Mol. Pharmaceutics, 2013, 10, 1998-2007
5. I.A. Kaltashov, C.E. Bobst, S.N. Nguyen, S. Wang. Emerging mass spectrometry-based approaches to probe protein-receptor interactions: focus on overcoming physiological barriers. Adv. Drug Deliv. Rev., 2013, 65, 1020-1030
6. C.E. Bobst, S. Wang, W.-C. Shen, and I.A. Kaltashov. Mass spectrometry study of a transferrin-based protein drug reveals the key role of protein aggregation for successful oral delivery. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 13544-13548