Research Projects
ClyA toxinOmpG sensorNanopore translocation
The non-classic attack pathway of E. coli ClyA pore-forming toxin
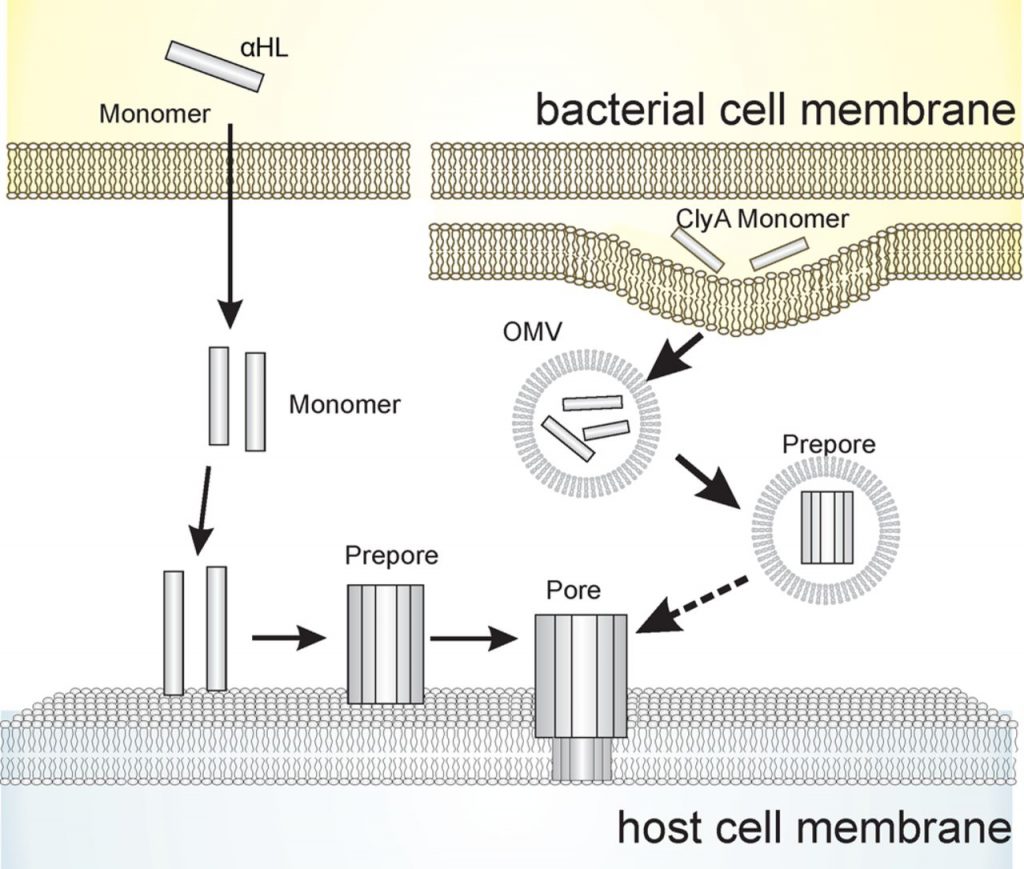
We are interested in unravel the mechanism by which ClyA toxin attack host cells. Cytolysin A (ClyA) from E. coli has recently emerged with an atypical assembly pathway. Unlike most of pore-forming toxins that are released directly into the extracellular environment, ClyA is secreted in an encapsulated form within outer membrane vesicles (OMVs) that bud from the outer membrane of bacteria.Questions to be answered include:
1) What is the structural conformation of ClyA in OMVs;
2) How do ClyA monomers recruit each other to from functional oligomers.
3) What is the pathway for the ClyA toxin to enter its battlefield: host cellular membrane?
We are to reveal the unusual mechanism through structural and functional studies using a combination of biochemical and biophysical approaches, including fluorescence spectroscopy, single-molecular total internal reflection fluorescence microscopy (TIRF), crystallography and live cell imaging by confocal microscopy.
An OmpG nanopore tool kit for sensing disease markers
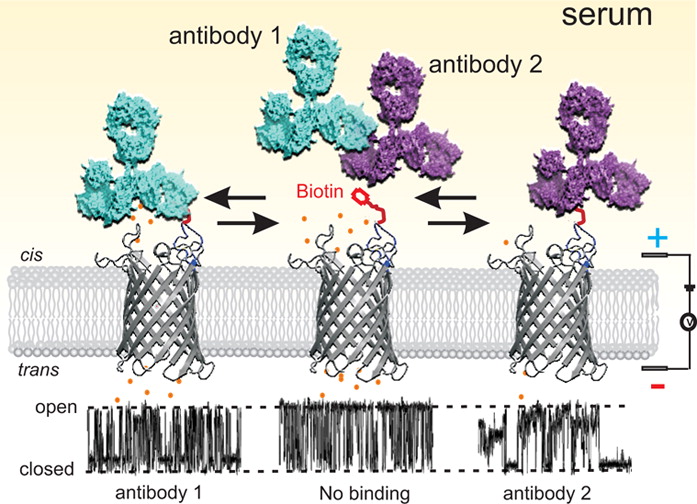
In Nanopore sensing, voltage drives analyte molecules through a single nanopore in the membrane that is surrounded in ionic solution. The ionic flow through the nanopore is temporarily blocked by various analytes to a different level. In turn, by measuring these characteristic ionic current blockades, the identities of the analytes can be obtained. Molecules that can be detected by nanopore range from metal ions, small organic polymers, peptides, proteins and nucleic acids.
Rapid, precise and early detection of biomarkers are essential for diagnosis of viral infection and cancer development. We aim to develop an OmpG nanopore sensor toolkit for applications in medical diagnostics and biodefense. We create our OmpG nanopore sensors by introducing a high-affinity recognition site to the highly flexible loops. The principle of our OmpG nanopore sensor is as such: catching a target protein by these loops results in the alteration of the ionic current. Most importantly we are building an OmpG tool kit containing millions of different sensors that can be adapt to detect any of the desirable protein biomarkers.
Molecular translocation through protein nanopores
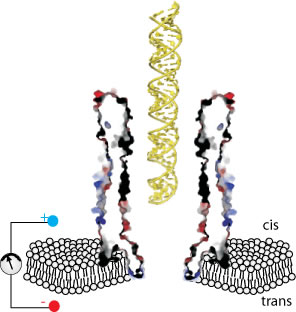
Molecular translocation through a nanopore is a fundamental phenomenon in nature, including the migration of mRNA through nuclear pores, injection of viral DNA or RNA into host cells, and the delivery of pathogenic proteins by bacterial type III and IV secretion systems. A detailed understanding of how polymers translocate through a nanopore not only provide new insight into natural processes but also aid the development of applications in high-resolution DNA sequencing as well as drug delivery.
Our group is working on a variety of biological nanopores that constitutes ionic channels, bacterial porins and toxins. We investigate the forces and interactions governing the translocation of the molecules though these nanopores. Particularly we are interested in manipulating nanopores’ shape, pore size and surface property to tune the translocation velocity in order to develop the third-generation DNA sequencing technology. In the other application of using nanopores as therapeutics to treat cancer, we aim to design nano-delivery devices that can directly and specifically courier drug molecules across the cell membrane of cancer cells.