Hydrogen exchange reveals a stable and expandable core within the aspartate receptor cytoplasmic domain.
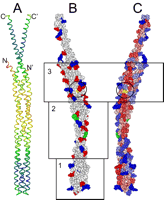
Murphy, O. J., III, Yi, X., Weis, R. M., & Thompson, L. K. (2001) “Hydrogen exchange reveals a stable and expandable core within the aspartame receptor cytoplasmic domain“, J. Biol. Chem. 276, 43262-43269.