Site-directed rotational resonance solid-state NMR distance measurements probe structure and mechanism in the transmembrane domain of the serine bacterial chemoreceptor.
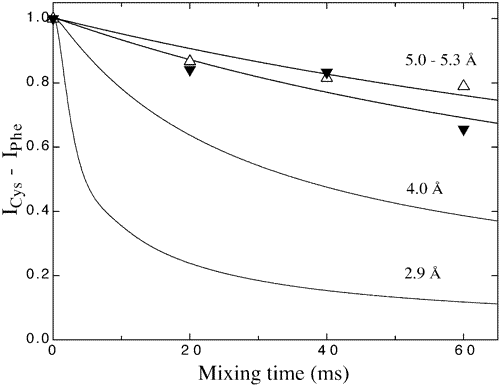
Isaac, B., Gallagher, G. J., Balazs, Y. S., & Thompson, L. K., (2002) “Site-directed rotational resonance solid-state NMR distance measurements probe structure and mechanism in the transmembrane domain of the serine bacterial chemoreceptor“, Biochemistry 41, 3025-3036.