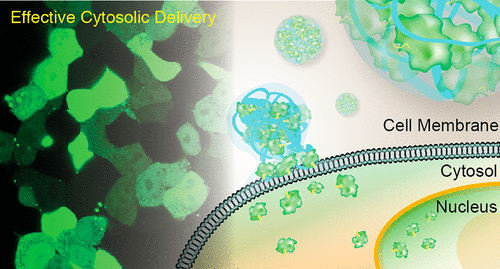
Access to the cytosol is a fundamental requirement for the efficacy of cell-based therapeutics. Nanocarrier technologies promote cellular uptake, but the vast majority rely on inefficient endosomal uptake/release pathways.
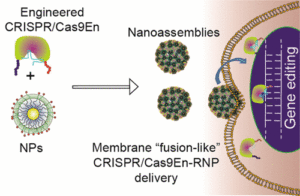
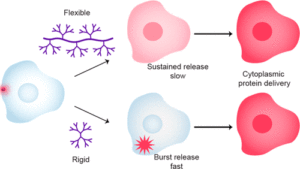
Our research harnesses the power of supramolecular chemistry and bioconjugation to enable direct cytosolic delivery of biomacromolecules. We utilize programmable nanotechnologies, including polymers and gold nanoparticles, to generate highly versatile supramolecular delivery vehicles capable of unique cellular interactions and featuring therapeutically relevant pharmacokinetic properties.
Selected References:
- Adv. Funct. Mater., 2024
- Chem. Soc. Rev., 2023
- Nanoscale, 2023
- ACS Nano, 2023
- ACS Nano, 2022
- J. Am. Chem. Soc. 2020
- J. Am. Chem. Soc. 2021
- Advanced Therapeutics.
- ACS Nano. 2017
- ACS Appl. Mater. Interfaces.
