Array-based (Chemical nose) sensing
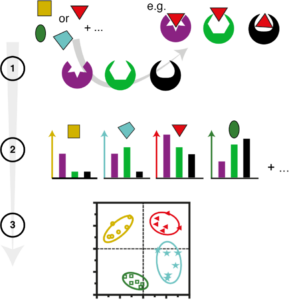
Research in sensing subgroup in the Rotello Group focuses on the design of hypothesis-free array-based “chemical nose” sensor for a broad range of biological sensing applications. A “chemical nose” sensor is broadly defined as an array-based system that uses synthetic molecules and/or materials to mimic the mammalian olfactory system. Unlike specific sensing, the chemical nose works on the principle of selective binding between an analyte and an array of cross-reactive receptors to generate distinct responses for each analyte and hence, the system is hypothesis-free. The responses can then be read out and linked back to the analyte through pattern recognition (Figure 1). Array-based sensing strategies can maximize sensitivity, achieving the detection of subtle changes in complex patterns. In the Rotello lab, we work on developing multi-channel sensor array to achieve multidimensional, high-content output, rendering high-throughput screening.
Figure 1. An overview of chemical nose sensing: Component in a mixture interacts in different ways with elements of a cross-reactive array. The transduction of the interactions leads to pattern generation for the combination of elements. The patterns are then processed, and it is possible to detect more analytes than there are elements.
Cell-based sensing application
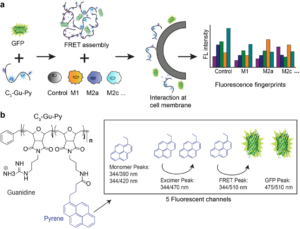
Cell phenotyping is emerging as an important tool for understanding and treating diseases, with rapid assessment of cell-behavior in response to environmental stimuli. Therefore, one key area of sensing is to develop rapid sensors for cell phenotyping. As an example, our lab recently reported the development and application of a polymer–protein supramolecular assembly as a sensor array to gather high-throughput, high-content information on macrophage polarization state. The sensor is composed of only two elements: a guanidine-functionalized cationic polymer, and an anionic green fluorescent protein (GFP). The two entities form a complex through electrostatic interactions, resulting in a Förster resonance energy transfer (FRET) pair. When this sensor is applied to macrophages in different polarization/sub-polarization states, it yields fluorescent signals in five channels. The multidimensional output is then quantitatively analyzed using linear discriminant analysis (LDA) to reproducibly classify different macrophage activation states (Figure 2).
Figure 2. Schematic illustration of phenotyping macrophage activation states using array-based sensor. (a) FRET-based sensor assembly was formed between PONI–C3-guanidine-pyrene and GFP. (b) Chemical structure of PONI–C3-guanidine-pyrene and the resulting five fluorescence channels in the FRET complex.
Serum-based sensing application
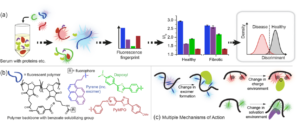
Point of care (PoC) diagnostics based on blood serum allow rapid and accurate diagnosis of more disease states than any other body fluid, and can be administered at the hospital bedside. Serum is however a challenging medium for sensors, containing thousands of different proteins, as well as salts, carbohydrates, and lipids. Serum-based PoC diagnostics must be quick, robust, low-cost and use small sample volumes of serum. In a recent work, our lab has extended the use of array-based sensing using polymers into rapid and robust diagnostic for liver fibrosis (Figure 3). By mixing three of the chemically stable polymers in a single, multiplexed array, an information-rich output (four fluorescent channels) is generated from a single sample measurement. This array can accurately distinguish non-fibrotic patients from those with early-stage liver fibrosis. Significantly, the polymer sensor does not degrade in ambient conditions, dramatically increasing the viability of this platform for PoC diagnostics relative to the biologicals used in current methods.
Figure 3. Liver fibrosis diagnosis using array-based sensing strategy. a) Schematic illustration of polymer-based array sensing for serum proteome to distinguish between fibrotic and nonfibrotic patients. b) Polymer structure featuring three responsive fluorophores. c) Potential working principle of environmental polymers.
In the ongoing studies, we are exploring both novel sensor platforms as well as interesting biological systems to apply this methodology. Current projects feature in synthetic material toxicity, liver disease and heart disease etc.
Selected References: